Have you experienced pain, implant failure, or revision surgery after receiving a Cartiva Synthetic Cartilage Implant? You may be entitled to compensation. The attorneys at Wilentz, Goldman & Spitzer, P.A. are investigating Cartiva lawsuits nationwide—contact us today for a free case evaluation.
Cartiva Implant Recall: What You Need to Know — and How to Protect Your Rights
The Cartiva Synthetic Cartilage Implant (SCI) was promoted as a breakthrough for people suffering from arthritis in the big toe joint—offering pain relief and mobility without joint fusion. But thousands of patients now report severe pain, implant failure, and costly revision surgeries. The FDA has issued a recall, and lawsuits are underway against the manufacturer.
If your Cartiva implant failed or caused complications, you may be entitled to compensation.
Contact Wilentz, Goldman & Spitzer, P.A. for a free consultation today.
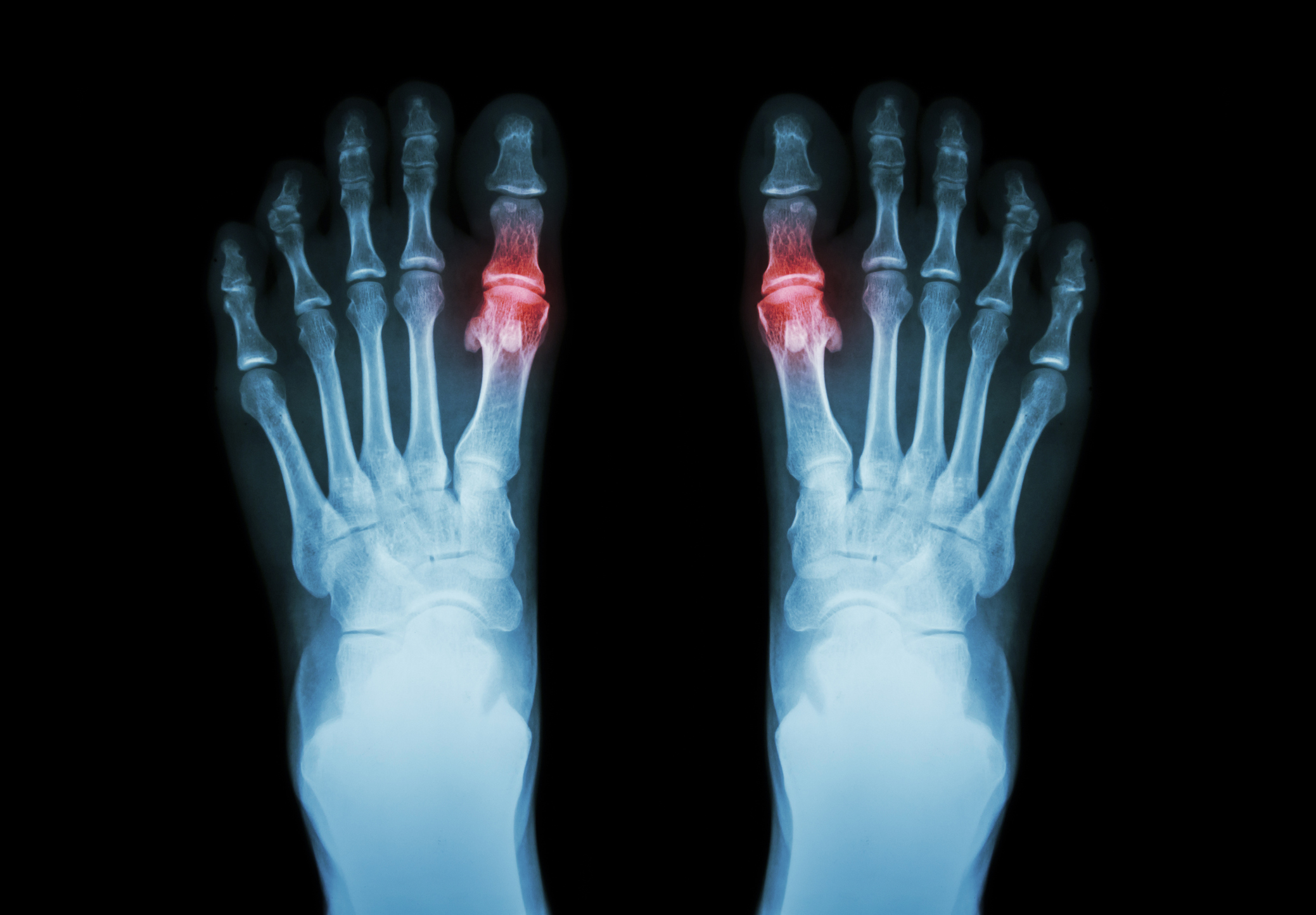
About the Device
Approved by the FDA in 2016, the Cartiva SCI was made from a hydrogel meant to act like natural cartilage—absorbing shock and protecting the joint. But despite its promises, real-world results tell a different story: patients have reported premature breakdown, displacement, and worsening pain that often leads to fusion surgery.
Experiencing pain or stiffness after a Cartiva implant?
Our legal team can review your situation at no cost.
Possible Cartiva Injuries & Complications
Common Cartiva Implant Complications Reported by Patients
- Implant Failure — Device breakdown requiring removal or replacement
- Chronic Pain — Pain returns or worsens after surgery
- Implant Displacement — Device moves out of position, reducing stability
- Loss of Motion — Joint stiffness or fusion
- Revision Surgery — Painful and expensive corrective procedures
Recent studies show failure rates as high as 79%, prompting a full FDA recall of all Cartiva Synthetic Cartilage Implants manufactured from 2016 onward.
If your Cartiva implant has failed, you are not alone—and you may have a claim.
Take the Next Step Toward Justice
If you or someone you love received a Cartiva implant and later suffered pain, failure, or needed additional surgery, you may be eligible for compensation.
Our firm has decades of experience handling defective medical device and product liability cases. We represent clients nationwide and work on a contingency fee basis—you pay nothing unless we win.
