The Cartiva Synthetic Cartilage Implant (SCI) was designed as a groundbreaking solution for individuals with arthritis in the big toe joint. Marketed as a durable and minimally invasive alternative to joint fusion, the Cartiva implant promised pain relief and improved mobility. However, reports from patients and surgeons suggest that the device may not perform as intended, leading to an FDA recall and Cartiva lawsuits.
The Cartiva SCI was approved by the FDA in 2016. The implant is made of a hydrogel material designed to mimic natural cartilage and cushion the big toe joint. Unlike traditional joint fusion surgery, which limits motion, the Cartiva implant was intended to maintain mobility while alleviating pain.
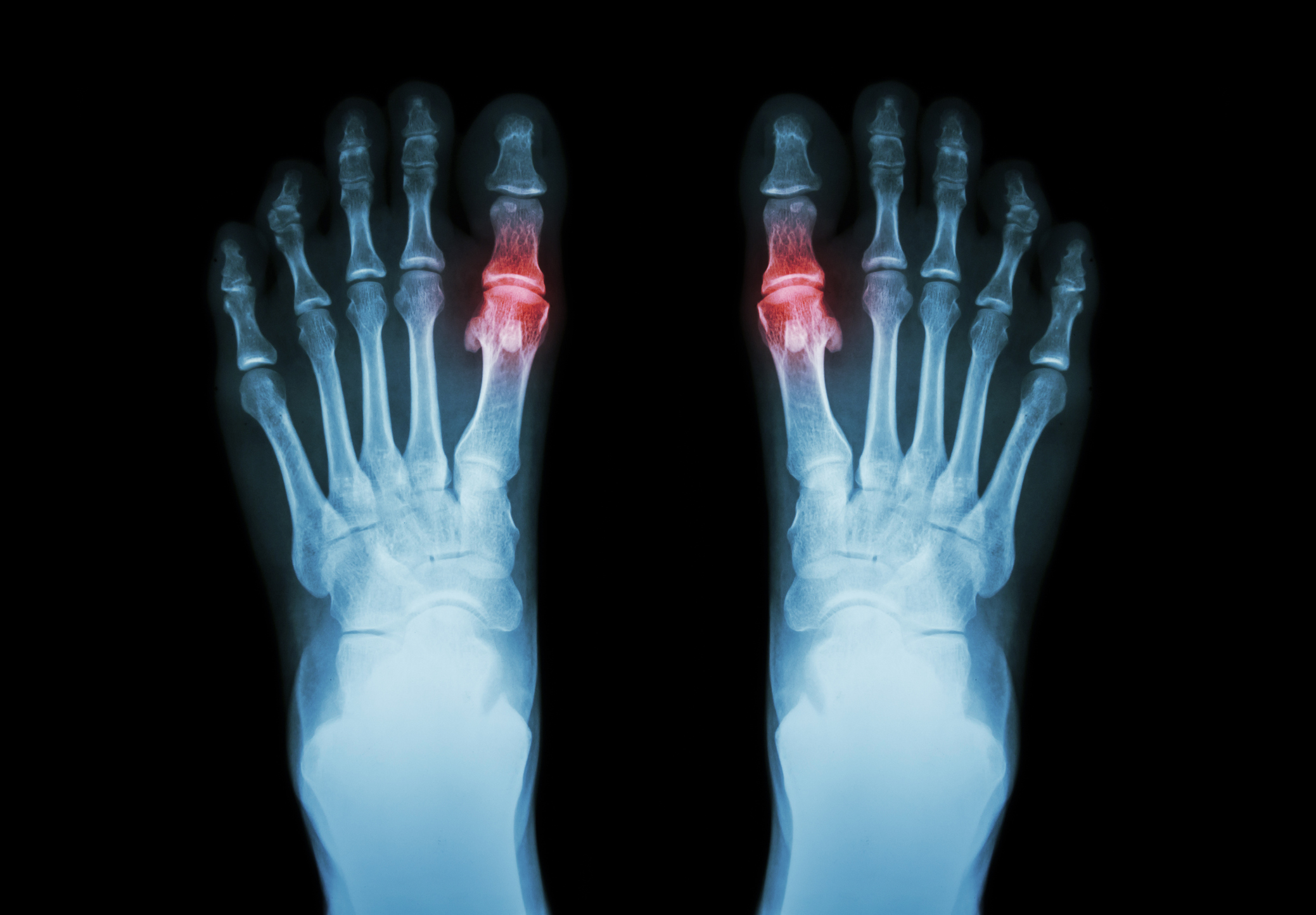
Despite its intended benefits, studies have shown a much higher failure rate than initially reported by its manufacturer. Patients report complications following Cartiva implantation, including:
With some studies showing a 79% failure rate, the FDA had little choice but is issue a federal recall of all Cartiva Synthetic Cartilage Implants manufactured from 2016 to the present. If you or a loved one has been injured by the Cartiva SCI, please contact Wilentz, Goldman & Spitzer, P.A. immediately for a free consultation.
Lawsuits are being filed by patients who allege that the Cartiva implant was defectively designed, inadequately tested and that the manufacturer failed to warn patients and surgeons about the risks. Some of the key legal claims in these cases include:
Many cases are now proceeding in state and federal courts. It remains to be seen whether individual lawsuits will be consolidated into a multi-district litigation (MDL) or class action lawsuit.
Wilentz, Goldman & Spitzer, P.A. is actively investigating Cartiva implant cases and is committed to holding negligent medical device manufacturers accountable. Contact us today for a free consultation to discuss your legal rights.
Navigating the complexities of medical device litigation can be overwhelming. We've compiled answers to some common questions to help you understand your options if you've been affected by a defective Cartiva implant.
A: Yes, absolutely. While our firm is based in New Jersey, we proudly represent Cartiva implant patients from all over the country. Our legal team is equipped to handle cases across state lines, and our New Jersey location, the same state as defendant Stryker, provides us with unique advantages in this litigation. We are dedicated to advocating for injured individuals nationwide.
A: It is unlikely. While each state has its own statute of limitations, generally speaking, the window for filing these lawsuits has not expired for most individuals, especially given the October 2024 recall of Cartiva implants distributed since July 2016. The specific timing can depend on when you first became aware of your implant's issues or when the recall occurred. If you are concerned about the timing of your potential lawsuit, please contact us for a free, confidential consultation. We can assess your specific circumstances and advise you on the applicable deadlines.
A: No, these cases do not involve suing your doctor. Cartiva lawsuits focus on holding the manufacturer, Stryker Corporation, responsible for injuries and losses caused by a defective medical device. At Wilentz, we understand and respect the important relationship you have with your healthcare provider. We work alongside your doctors by gathering necessary medical records and understanding the impact of your injury, all in pursuit of justice against the device manufacturer.
A: Patients with Cartiva implants have reported a range of significant complications, including:
If you are experiencing any of these symptoms after receiving a Cartiva implant, it is crucial to seek medical attention and then consider your legal options.
A: If successful, a Cartiva implant lawsuit can help you recover various types of damages. These may include compensation for your past and future medical expenses related to the defective implant (including revision surgeries), lost wages due to time off work, pain and suffering, loss of enjoyment of life, and other related financial and non-financial losses. The specific compensation you may be entitled to will depend on the unique details of your case.
A: We typically handle Cartiva implant lawsuits on a contingency fee basis. This means you pay no upfront fees, and we only get paid if we successfully recover compensation for you, either through a settlement or a verdict. Our fees are a percentage of the recovery, so you don't have to worry about out-of-pocket legal expenses while pursuing your claim.
A: The timeline for medical device litigation can vary significantly based on the complexity of the cases, the number of plaintiffs, and whether the cases are consolidated into a Multi-District Litigation (MDL). While some cases may settle sooner, it is not uncommon for complex defective medical device lawsuits to take several years to resolve. We are committed to keeping our clients informed throughout every stage of the legal process.
A: Your health is paramount. First, seek medical attention from your doctor or a qualified orthopedic surgeon to address your symptoms and determine the appropriate medical course of action. Once your immediate medical needs are addressed, contact our firm for a free consultation. We can evaluate your situation and discuss your legal rights and options.
Joshua S. Kincannon
Shareholder
Lynne M. Kizis
Co-Chair, Mass Tort/Class Action Team
Shareholder