In June 2021, the U.S. Food and Drug Administration announced a recall of all Exactech Connexion GXL hip replacement liners due to wear-related issues of the polyethylene. There were approximately 89,050 Connexion GXL hip liners implanted between 2008 to 2019 in the United States. These implants have a risk of premature wear that could lead to health risks and require corrective revision surgery. Read the Exactech recall notice to healthcare professionals.
As medical technology advances and our population ages, those suffering from arthritis and other chronic joint problems increasingly seek relief by undergoing hip replacement surgery. For many, hip implant surgery is uneventful and patients make a full recovery with a new hip that they can expect to survive for at least ten years. Others do not fare as well and do not recover fully following surgery. They experience pain, swelling, limited range of motion, leg length discrepancy, and other complications -- that oftentimes lead to more surgery.
The GXL liner, part of a metal-on-polyethylene or metal-on-plastic hip implant was “enhanced” to “provide 59% wear reduction,” which should have resulted in greater longevity to provide a lifelong implant. However, the GXL liners have demonstrated the opposite outcome. Instead, the liners have had a higher rate of premature plastic wear in patients and early implant failure, ultimately leading patients to need revision surgery.
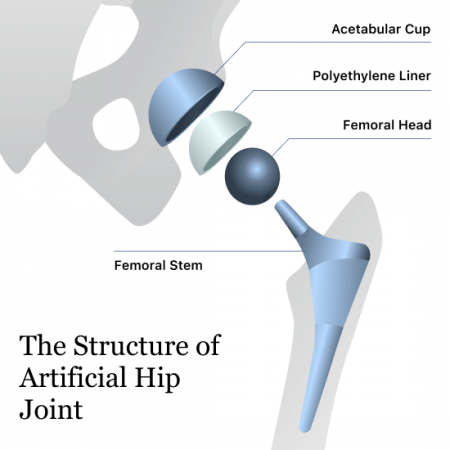
According to the recall notice, patients who have had a GXL hip implant in the last 6 years and who have not seen their surgeon in 12 months should see their doctor for a clinical exam and x-rays.
New Jersey recognizes that defective implants exist and has laws that allow injured patients to be compensated for their pain and suffering. Product liability cases may be brought on a preponderance of the evidence for a failure to warn, a manufacturing defect, or a design defect. The New Jersey Product Liability Act states:
“A manufacturer or seller of a product shall be liable in a product liability action only if the claimant proves by a preponderance of the evidence that the product… was not reasonably fit, suitable or safe for its intended purpose because it: a. deviated from the design specifications, formulae, or performance standards of the manufacturer or from otherwise identical units manufactured to the same manufacturing specifications or formulae, or b. failed to contain adequate warnings or instructions, or c. was designed in a defective manner.”
The New Jersey Supreme Court held in 1998 that in order to establish a claim of defective design, the Plaintiff “must prove . . . that the product could have been designed in an alternative manner so as to minimize or eliminate the risk of the harm.” Lewis v. Am. Cyanamid Co., 155 N.J. 544, 570, 715 A.2d 967, 980 (1998). Furthermore, The New Jersey Product Liability Act requires a manufacturer to produce an “adequate product warning.” N.J.S.A. § 2A:58C-4.
“An adequate product warning or instruction is one that a reasonably prudent person in the same or similar circumstances would have provided with respect to the danger and that communicates adequate information on the dangers and safe use of the product, taking into account the characteristics of, and the ordinary knowledge common to, the persons by whom the product is intended to be used, or in the case of prescription drugs, taking into account the characteristics of, and the ordinary knowledge common to, the prescribing physician.”
N.J.S.A. § 2A:58C-4, (emphasis added). Adequate product warnings warn of risks that manufacturers knew or should have known at the time the device was produced.
Lynne M. Kizis
Co-Chair, Mass Tort/Class Action Team
Shareholder